Life depends on precise, sequence-controlled polymer synthesis by the ribosome, a natural molecular machine that reads genes and writes the corresponding proteins by concatenating amino-acid building blocks. Our ambition is to create a synthetic ribosome, a molecular machine capable of translating a synthetic genetic code into a completely synthetic, sequence-defined polymer.
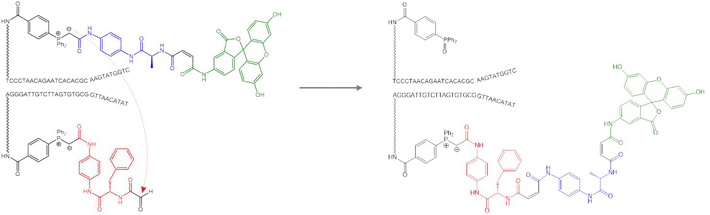
DNA-templated synthesis1
Our research is based on the principle of DNA-templated synthesis.1,2 Building blocks for polymer synthesis are attached to DNA adapters (analogous to tRNAs) that serve to identify them. These adapters are used by our molecular machinery to select building blocks and bring them into proximity, enabling their concatenation in a programmed sequence.
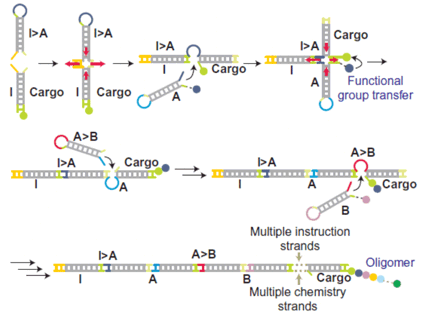
Autonomous, programmed synthesis3
With Rachel O'Reilly's group (Birmingham Chemistry) we have demonstrated programmed bond formation using both natural and unnatural backbone chemistries (peptide and Wittig, respectively), including sequence-programmed synthesis of 10-unit oligomers4 and the autonomous creation of DNA-tagged combinatorial libraries.3 We are developing better ways to integrate the chemistry of bond formation with the operation of the underlying DNA machinery to improve yield and reduce synthesis errors.
Genetically programmed synthesis by molecular machinery will allow exploration of vast new regions of chemical space by selection and evolution using libraries of products (1012 ~ 1015) created from combinatorial libraries of genes. This will open up many exciting applications including the directed evolution of non-natural polymers to match and extend the functionalities of peptides and proteins. Molecular machinery for programmed synthesis will also provide a platform to recreate complex biological behaviours such as gene regulation, bringing the creation of artificial living systems a step closer.
1. M.L. McKee et al., Angew. Chem. Int. Ed. 49, 7948 (2010)
2. C.T. Calderone, et al., Angew. Chem. Int. Ed. 41, 4104-8 (2002)
3. W. Meng et al., Nature Chem. 8, 542-548 (2016)
4. P.J. Milnes et al., Chem. Commun. 48, 5614-6 (2012)