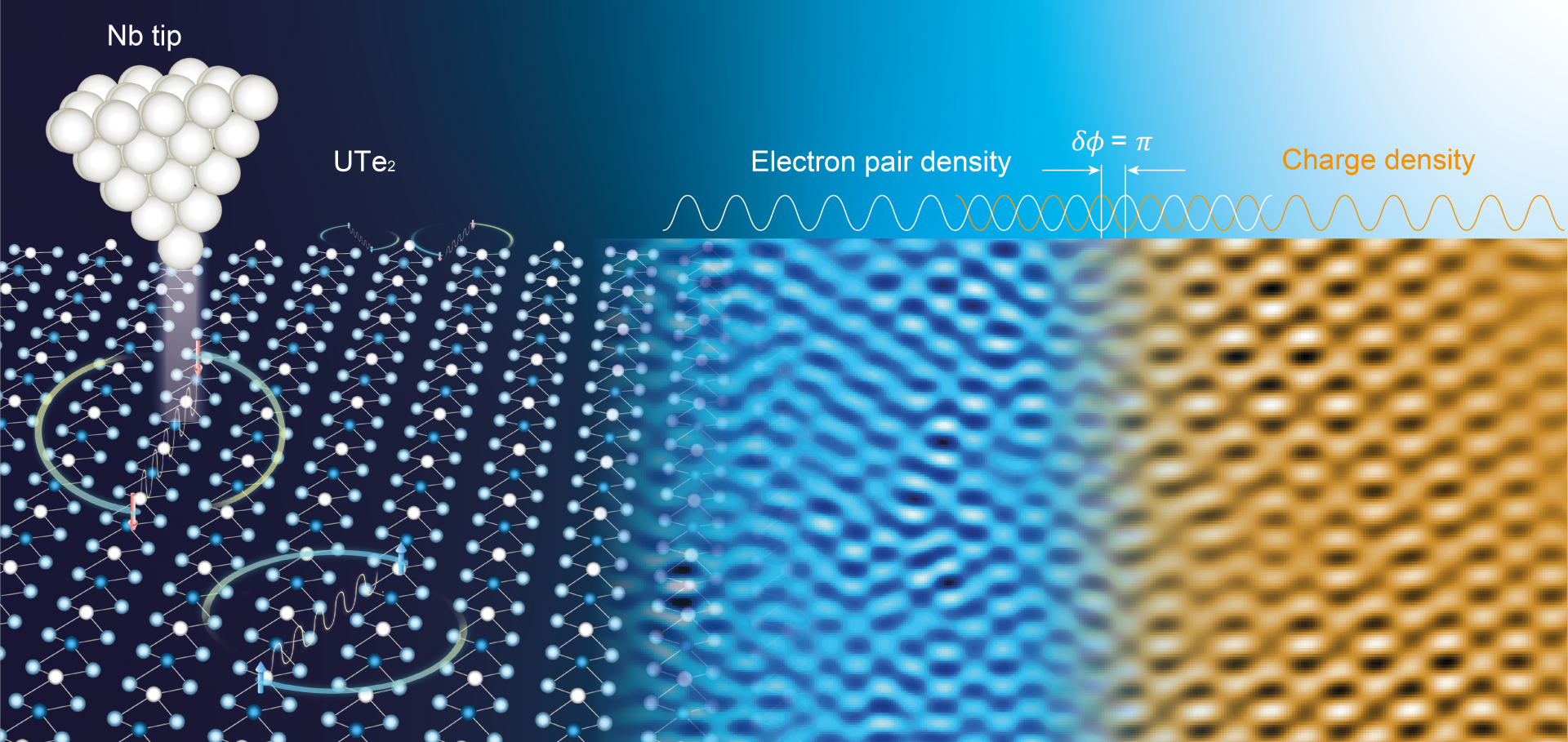
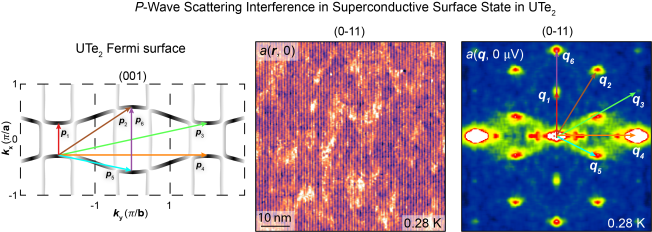
Pair wave function symmetry in UTe2 from zero-energy surface state visualization
Abstract:
Although nodal spin-triplet topological superconductivity appears probable in uranium ditelluride (UTe2), its superconductive order parameter Δk remains unestablished. In theory, a distinctive identifier would be the existence of a superconductive topological surface band, which could facilitate zero-energy Andreev tunneling to an s-wave superconductor and also distinguish a chiral from a nonchiral Δk through enhanced s-wave proximity. In this study, we used s-wave superconductive scan tips and detected intense zero-energy Andreev conductance at the UTe2 (0-11) termination surface. Imaging revealed subgap quasiparticle scattering interference signatures with a-axis orientation. The observed zero-energy Andreev peak splitting with enhanced s-wave proximity signifies that Δk of UTe2 is a nonchiral state: B1u, B2u, or B3u. However, if the quasiparticle scattering along the a axis is internodal, then a nonchiral B3u state is the most consistent for UTe2.