Molecular Ions and Quantum Technology
Trapped molecules are an exciting platform for the extending of quantum information science. They possess additional degrees of freedom into which it is convenient to encode information using resonant microwave fields. They are also able to interact resonantly across long distances due to their permanent electric dipole moments.
However, they are extremely sensitive to the trapping environment. Ion traps, on the other hand, offer a pristine environment for the control of quantum systems, but until now studies into using molecular ions as a quantum resource have been limited.
This project explores this space, and is the only one in the world attempting to use the radical SrF as a starting point. SrF is a special molecule, as it can be laser cooled we can prepare the internal state exceptionally well. By ionising it with resonant lasers we can preserve this state preparation and perform quantum operations on the molecule using microwaves. The state of the molecule will be read out using the high-fidelity techniques developed in Oxford on a single Sr+ ion. Ultimately, the goal is to realise a quantum gate between pairs of molecules and to use this to perform a quantum operation.
This project will develop novel ion trap technology, a state-of-the-art molecular beam and next-generation laser sources and optics. We are always looking for the best students to take this further.
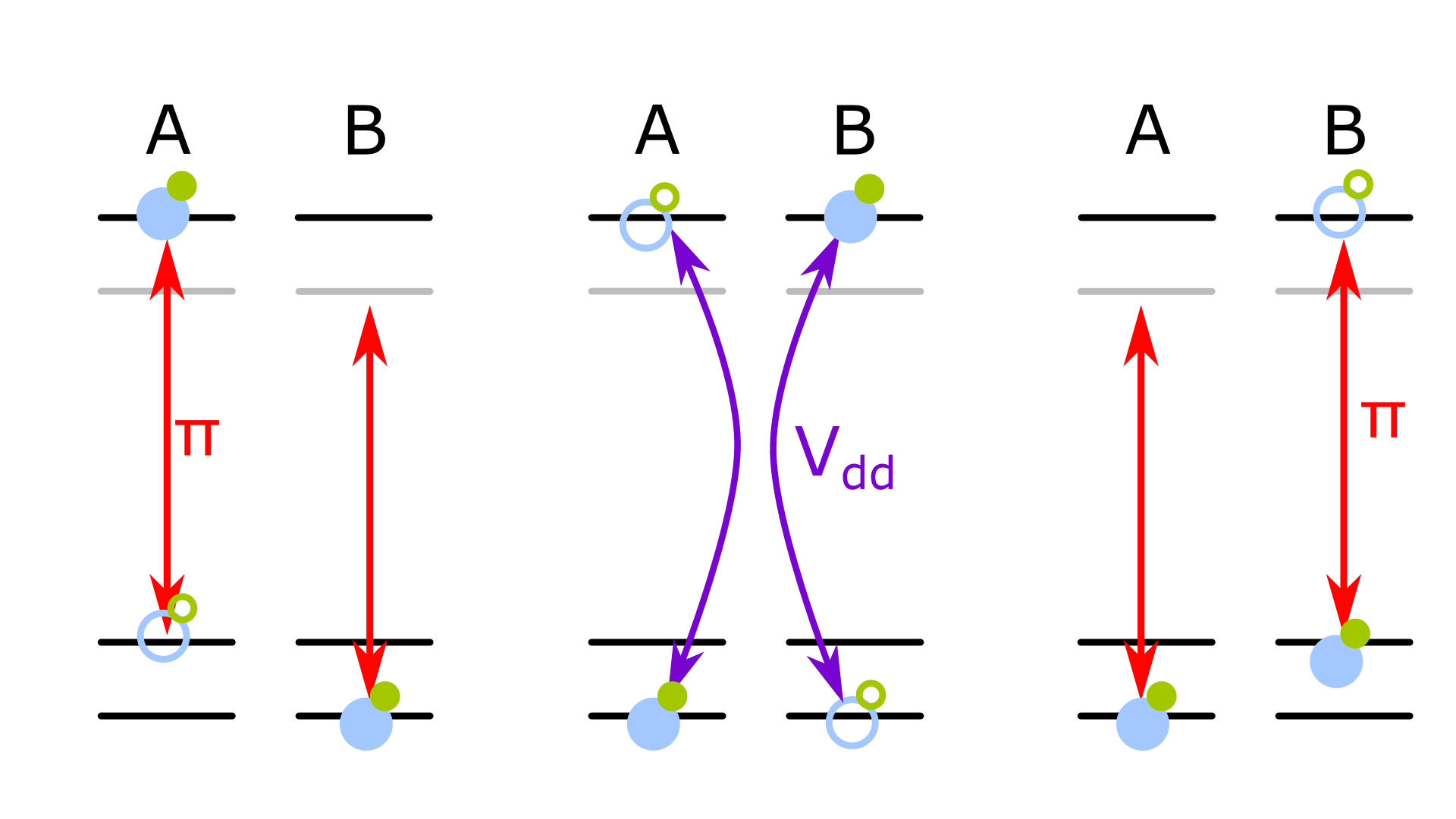
Using the dipole-dipole interaction to implement an iSWAP gate between molecules. A resonant microwave field excites one molecule, which interacts with its neighbour via the dipole-dipole interaction, this causes the excitation to switch between molecules. A second microwave pulse de-excites the second molecule back into the upper logic level, completing the gate.
Contact
Jacob Blackmore: jacob.blackmore@physics.ox.ac.uk
