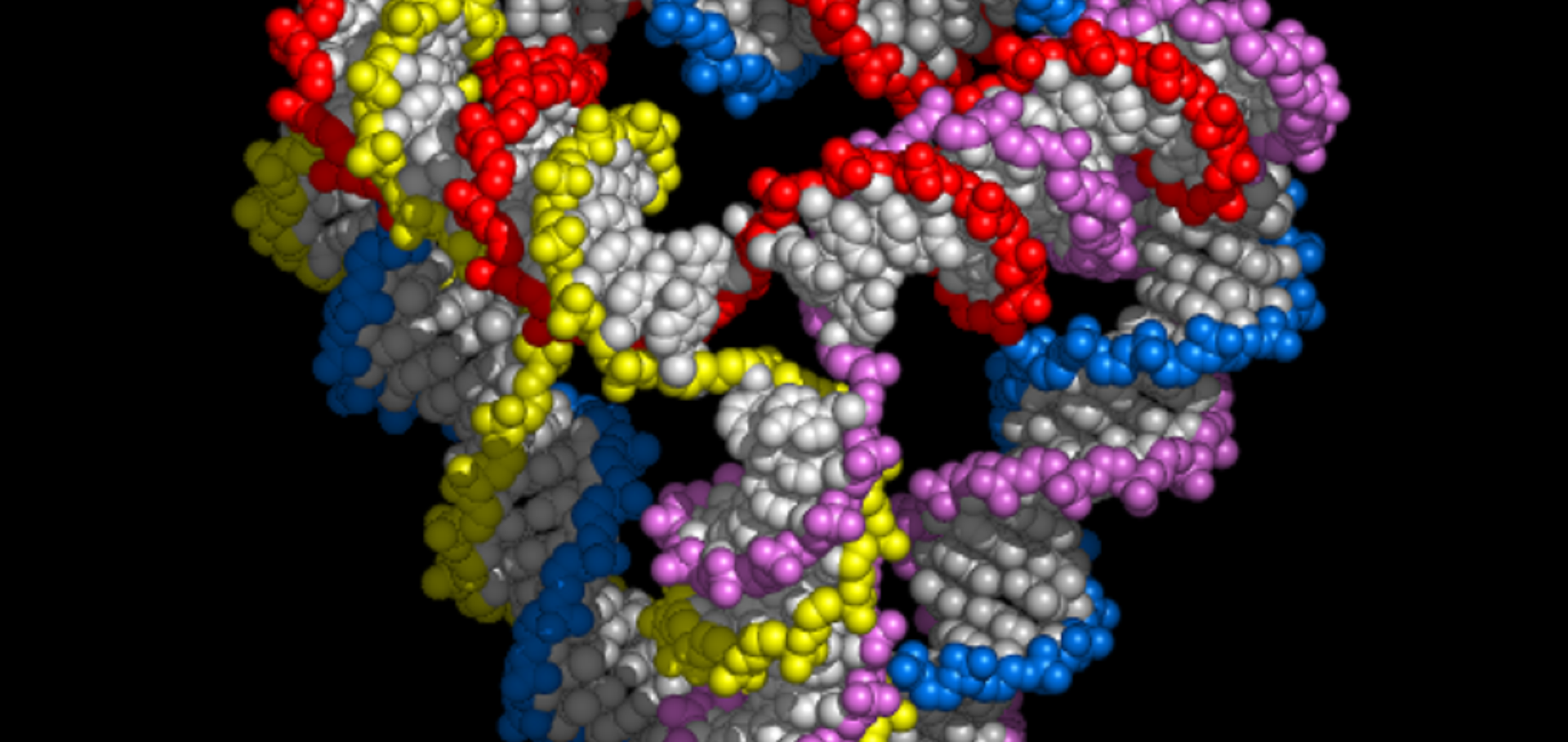
Guiding the folding pathway of DNA origami
Nature Springer Nature 525:7567 (2015) 82-86
DNA walker circuits: computational potential, design, and verification
Natural Computing 14:2 (2015) 195-211
Abstract:
Unlike their traditional, silicon counterparts, DNA computers have natural interfaces with both chemical and biological systems. These can be used for a number of applications, including the precise arrangement of matter at the nanoscale and the creation of smart biosensors. Like silicon circuits, DNA strand displacement systems (DSD) can evaluate non-trivial functions. However, these systems can be slow and are susceptible to errors. It has been suggested that localised hybridization reactions could overcome some of these challenges. Localised reactions occur in DNA ‘walker’ systems which were recently shown to be capable of navigating a programmable track tethered to an origami tile. We investigate the computational potential of these systems for evaluating Boolean functions and forming composable circuits. We find that systems of multiple walkers have severely limited potential for parallel circuit evaluation. DNA walkers, like DSDs, are also susceptible to errors. We develop a discrete stochastic model of DNA walker ‘circuits’ based on experimental data, and demonstrate the merit of using probabilistic model checking techniques to analyse their reliability, performance and correctness. This analysis aids in the design of reliable and efficient DNA walker circuits.DNA walker circuits: computational potential, design, and verification
Natural Computing Springer Nature 14:2 (2015) 195-211
Automated design and verification of localized DNA computation circuits
DNA 2015: DNA Computing and Molecular Programming Springer International Publishing Switzerland 9211 (2015) 168-180
Abstract:
Simple computations can be performed using the interactions between single-stranded molecules of DNA. These interactions are typically toehold-mediated strand displacement reactions in a well-mixed solution. We demonstrate that a DNA circuit with tethered reactants is a distributed system and show how it can be described as a stochastic Petri net. The system can be verified by mapping the Petri net onto a continuous time Markov chain, which can also be used to find an optimal design for the circuit. This theoretical machinery can be applied to create software that automatically designs a DNA circuit, linking an abstract propositional formula to a physical DNA computation system that is capable of evaluating it.Folding pathways: DNA origami as a model system
EUROPEAN BIOPHYSICS JOURNAL WITH BIOPHYSICS LETTERS 44 (2015) S67-S67