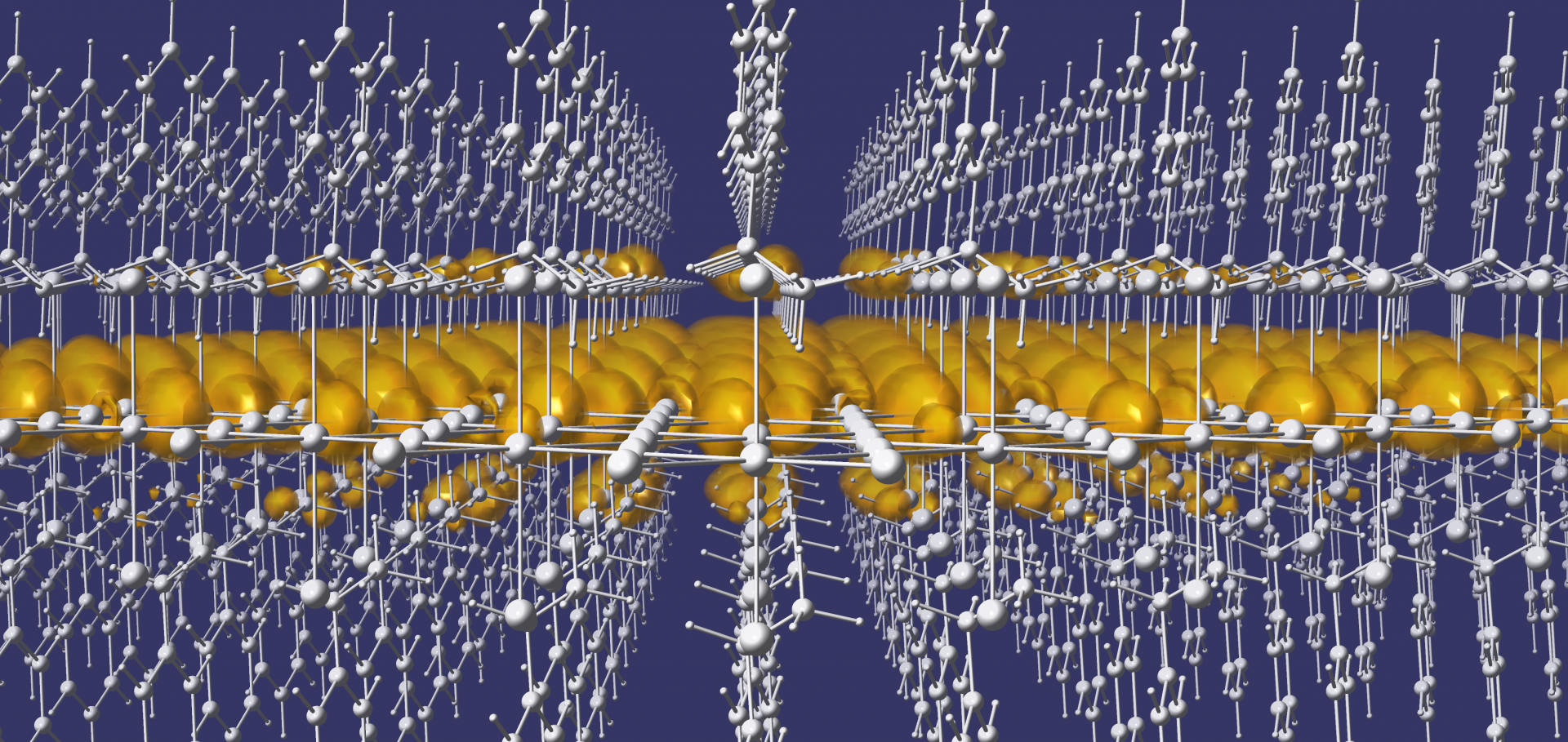
Hybrid Halide Perovskites: Fundamental Theory and Materials Design
Chapter in Handbook of Materials Modeling, Springer Nature (2020) 295-324
Excitonic Properties of Lead-Halide Perovskites from First Principles Computational Modeling
Fundacio Scito (2019)
Excitonic Properties of Lead-Halide Perovskites from First Principles Computational Modeling
Fundacio Scito (2019)
Hybrid Halide Perovskites: Fundamental Theory and Materials Design
(2018)
Cubic or orthorhombic? Revealing the crystal structure of metastable black-phase CsPbI3 by theory and experiment
ACS Energy Letters American Chemical Society 3 (2018) 787-1794