Perovskite based optoelectronics: molecular design perspectives – a themed collection
Molecular Systems Design & Engineering Royal Society of Chemistry (RSC) 3:5 (2018) 700-701
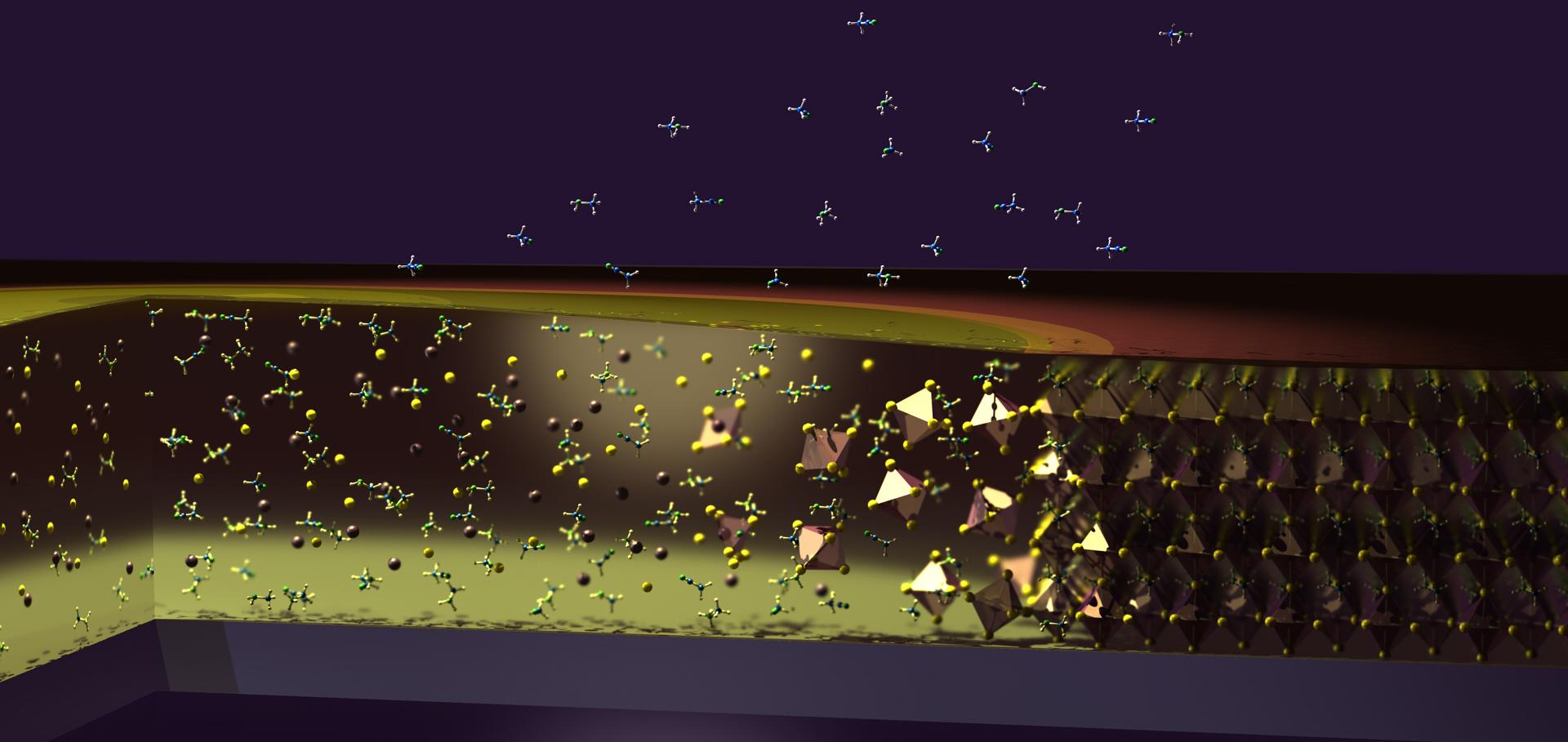
Unravelling the improved electronic and structural properties of methylammonium lead iodide deposited from acetonitrile
Chemistry of Materials American Chemical Society 30:21 (2018) 7737-7743
Abstract:
Perovskite-based photovoltaics are an emerging solar technology with lab scale device efficiencies of over 22 %, and significant steps are being made toward their commercialization. Conventionally high efficiency perovskite solar cells are formed from high boiling point, polar aprotic solvent solutions. Methylammonium lead iodide (CH3NH3PbI3) films can be made from a range of solvents and blends; however, the role the solvent system plays in determining the properties of the resulting perovskite films is poorly understood. Acetonitrile (ACN), in the presence of methylamine (MA), is a viable nontoxic solvent for fabrication of CH3NH3PbI3 photovoltaic devices with efficiencies >18 %. Herein we examine films prepared from ACN/MA and dimethylformamide (DMF) and scrutinize their physical and electronic properties using spectroscopy, scanning probe imaging, and ion scattering. Significant differences are observed in the chemistry and electronic structure of CH3NH3PbI3 films made with each solvent, ACN/MA produces films with superior properties resulting in more efficient photovoltaic devices. Here we present a holistic and complete understanding of a high performance perovskite material from an electronic, physical, and structural perspective and establish a robust toolkit with which to understand and optimize photovoltaic perovskites.Modification of the fluorinated tin oxide/electron-transporting material interface by a strong reductant and its effect on perovskite solar cell efficiency
Molecular Systems Design and Engineering Royal Society of Chemistry 3:5 (2018) 741-747
Abstract:
To date, the most efficient hybrid metal halide peroskite solar cells employ TiO2 as electron-transporting material (ETM), making these devices unstable under UV light exposure. Replacing TiO2 with fullerene derivatives has been shown to result in improved electronic contact and increased device lifetime, making it of interest to assess whether similar improvements can be achieved by using other organic semiconductors as ETMs. In this work, we investigate perylene-3,4:9,10-tetracarboxylic bis(benzimidazole) as a vacuum-processable ETM, and we minimize electron-collection losses at the electron-selective contact by depositing pentamethylcyclopentadienyl cyclopentadienyl rhodium dimer, (RhCp*Cp)2, on fluorinated tin oxide. With (RhCp*Cp)2 as an interlayer, ohmic contacts can be formed, there is interfacial doping of the ETM, and stabilized power conversion efficiencies of up to 14.2% are obtained.Atomic layer deposited electron transport Layers in efficient organometallic halide perovskite devices
MRS Advances Cambridge University Press 3:51 (2018) 3075-3084
Abstract:
Amorphous TiO2 and SnO2 electron transport layers (ETLs) were deposited by low-temperature atomic layer deposition (ALD). Surface morphology and x-ray photoelectron spectroscopy (XPS) indicate uniform and pinhole free coverage of these ALD hole blocking layers. Both mesoporous and planar perovskite solar cells were fabricated based on these thin films with aperture areas of 1.04 cm2 for TiO2 and 0.09 cm2 and 0.70 cm2 for SnO2. The resulting cell performance of 18.3 % power conversion efficiency (PCE) using planar SnO2 on 0.09 cm2 and 15.3 % PCE using mesoporous TiO2 on 1.04 cm2 active areas are discussed in conjunction with the significance of growth parameters and ETL composition.Highly crystalline methylammonium lead tribromide perovskite films for efficient photovoltaic devices
ACS Energy Letters American Chemical Society 3:6 (2018) 1233−1240